Flying under radar, DEBIO-1143 is quietly moving into late phase development. In clinical development since 2010, very little clinical data available so far.
Target and scientific rationale
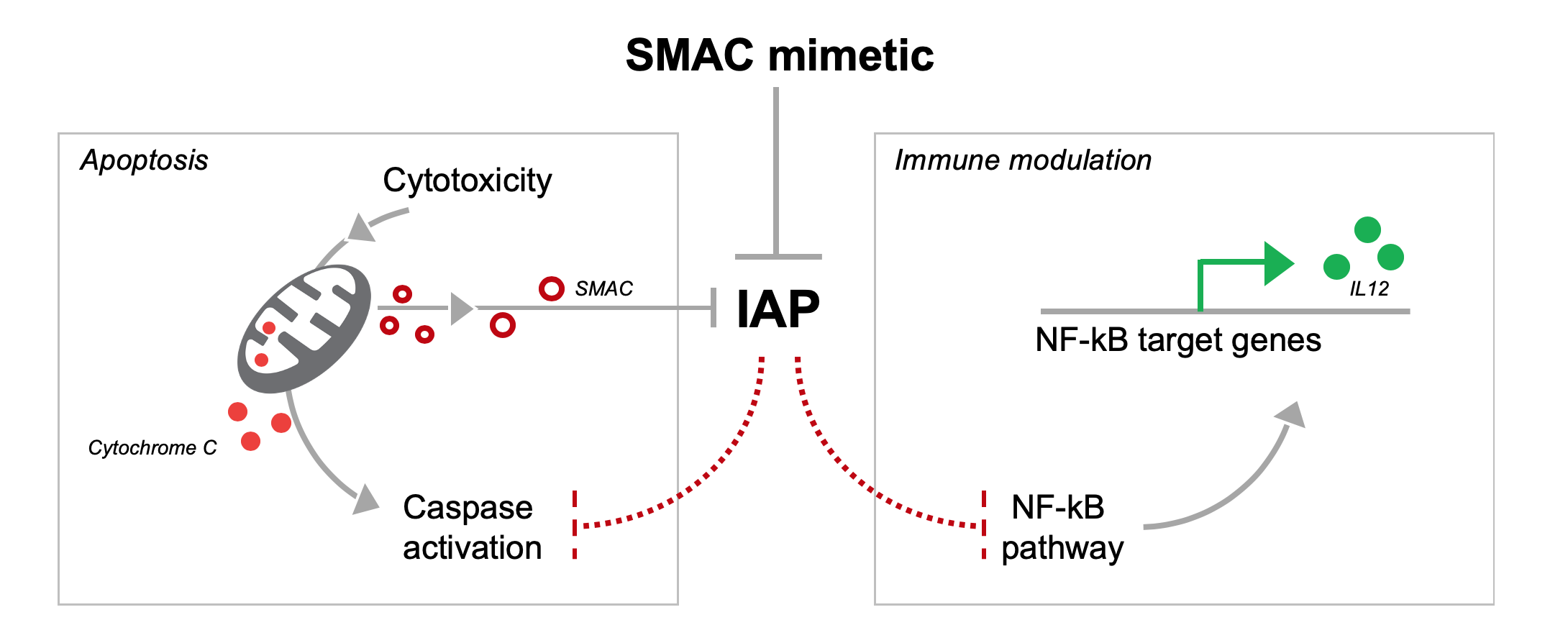
DEBIO-1143 is a small molecule SMAC mimetic, which functions as an IAP (inhibitor of apoptosis protein) antagonist. IAPs are negative regulators of caspase-dependent apoptosis: under cytotoxic stress, mitochondria release SMAC to disable IAPs and initiate caspase-dependent apoptosis. Like mitochondrial SMAC, SMAC mimetics facilitate caspase activation, even in tumor cells with high IAP expression.
Beyond apoptosis, IAPs also regulate inflammatory and immune responses, specifically through inhibition of the NF-kB-dependent T-cell activation. SMAC mimetics can reactivate the NF-kB pathway and target gene expression, including pro-inflammatory cytokine IL-12 (1)
Clinical development
Initial development by Ascenta Therapeutics; first-in-human study was initiated in 2010. In 2011, DEBIO-1143 (formerly AT‑406) was in-licensed by DebioPharm.
First clinical data was not reported until 2015. FIH data was published (2) and data from ph 1b/2 study combining DEBIO-1143 with paclitaxel/carboplatin was presented as a poster at AACR (3). This study was halted prior to enrollment in the extension cohorts (NSCLC, OvCa, MBC) due to “business decision” (NCT01930292). In 2016, a low key ESTRO poster presented the ph 1b portion of a randomized phase 2 study combining DEBIO-1143 with chemoradiation, this study was sponsored by the French cooperative group GERCOR (NCT02022098).
In 2020, DEBIO-1143 received FDA BTD for SCCHN in combination with chemoradiation.
A large phase 3 study, sponsored by DebioPharm and GORTEC, is planned in the same pt population (NCT04459715).
Wait, what?
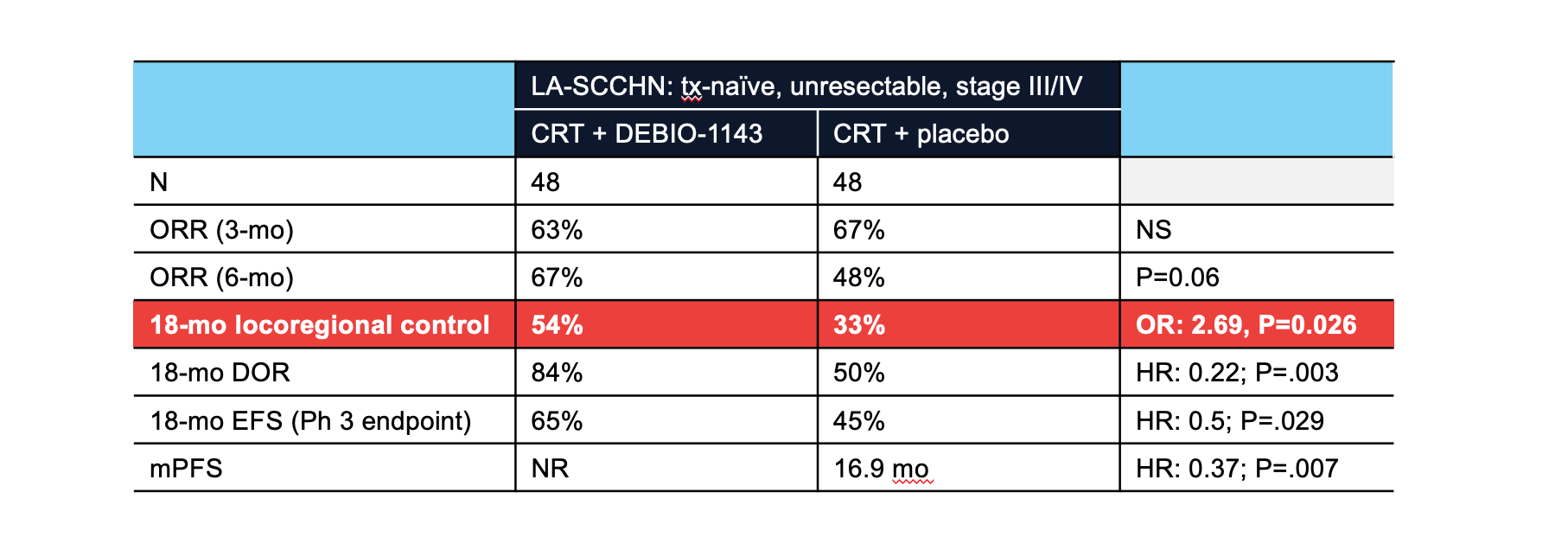
Data from the before mentioned GERCOR phase 2b study was presented as a LBA at ESMO 2019 (4). The study randomized 96 pts with locally advanced, unresectable SCCHN to cisplatin-based chemoradiation with or without DEBIO-1143. Primary endpoint of locoregional control at 18 months was 54% vs 33% (OR: 2.69, P=0.026); other endpoints also showed statistically significant improvements. Longer follow-up of this study will be presented at ESMO (LBA39). A large phase 3 study, sponsored by DebioPharm and GORTEC, is planned in the same pt population (NCT04459715).
Future development: IO combinations
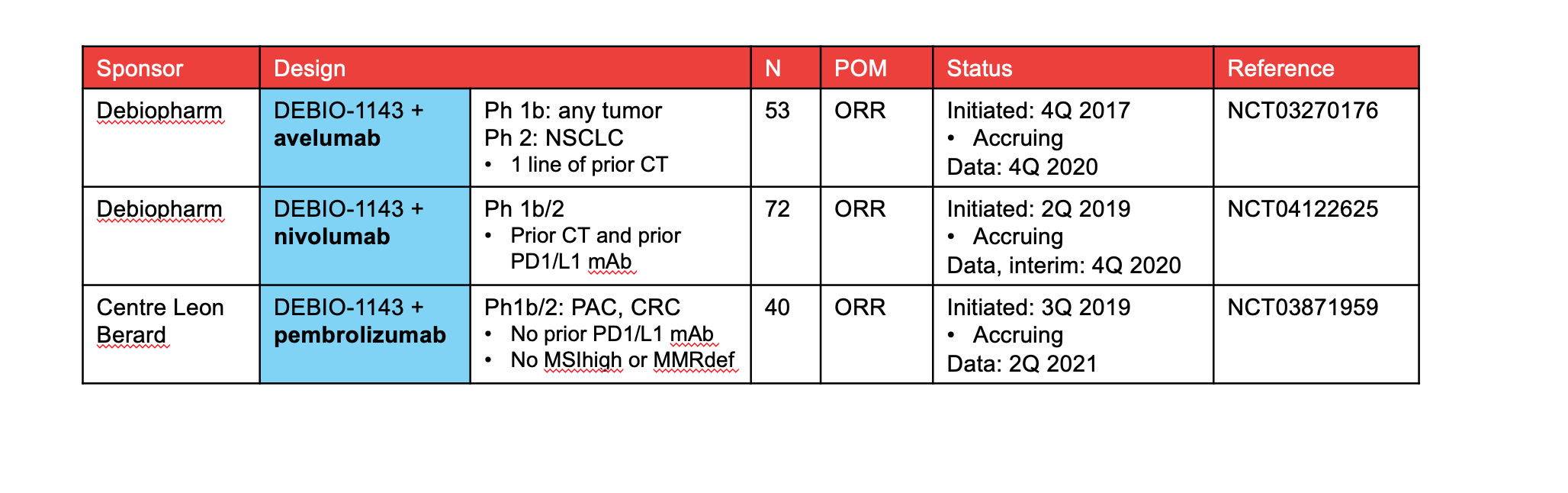
Debiopharm is exploring DEBIO-1143 combinations with immune checkpoint inhibitors. Early clinical data of DEBIO-1143 in combination with avelumab reported activity in NSCLC pts (5). Planned expansion cohort in NSCLC pts with 1 line of prior chemotherapy.
The competition
DEBIO-1143 is the first SMAC mimetics to make it to late phase development. Early competitors, LCL161 (Novartis) and birnanpant (Medivir), have recently dropped out of the pipeline. Of note: birinapant, in combination with pembrolizumab, showed a lack of response in CRC (press release); Medivir is currently looking to out-license birinapant.
Emerging competitors (all in phase 1b/2):
Astex: tolinapant (ASTX 660) in hematologic malignancies (NCT04155580, NCT04362007). FIH data was recently published (6)
Boehringer Ingelheim: BI 891065 with PD1 mAb in NSCLC extension cohort (NCT03166631)
Ascenta: APG-1387 with a PD1 mAb in 3 tumor cohorts (CRC, nasopharyngeal carcinoma, NSCLC; NCT04284488). Planned study with chemotherapy in PAC (China). FIH data presented at ASCO 2019 (7)
Novartis SMAC antagonist LCL161 has been in clinical development since 2008. The most recent clinical data showed activity, but also significant toxicity, in neoadjuvant TN BC (8). An ongoing basket study includes 3 cohorts combining LCL161 with spartalizumab (PD1 mAb) – no data has been presented (NCT02890069).
Reference
1. Beug et al., Nat Comm. 2017 (PMID: 28198370)
2. Hurwitz et al., Cancer Chemother Pharmacol. 2015 (PMID: 25716544)
3. Ray-Coquard et al., AACR 2015: C2
4. Bourhis et al.,ESMO 2019: LBA65
5. Juergens et al., ASCO 2019: 2599
6. Mita et al., Clin Cancer Res. 2020 (PMID: 31900279)
7. Rasco et al., ASCO 2019: 3125
8. Bardia et al., JCO. 2018 (PMID: 30235087)