
Janssen’s EGFR/MET bi-specific mAb (amivantamab) is developed in NSCLC pts with primary or acquired resistance to EGFR TKIs. Already, single agent amivantamab received FDA BTD for EGFR ex20in NSCLC. To expand beyond EGFR ex20in, Janssen is taking a bold approach by combining amivantamab with an unapproved, third generation EGFR TKI to take on single agent osimertinib in tx-naïve, EGFRmut NSCLC.
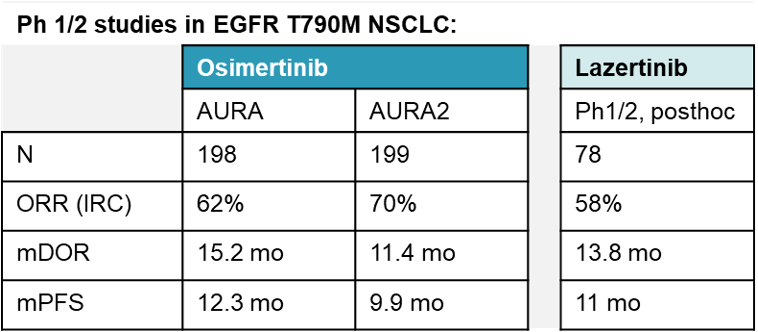
Designed as a third generation EGFR TKI, lazertinib entered clinical studies in 2017. In EGFR T790M NSCLC, ORR was 58% (by IRC), with a mDOR of 13.8 months and a mPFS of 11 months (ASCO 2020). These numbers fall within the range of those reported for another 3rd generation TKI, osimertinib (table), but also suggest that lazertinib is unlikely to beat osimertinib in a head-to-head study.
Yuhan has recently initiated a phase 3 study in treatment-naïve, EGFRmut NSCLC, comparing lazertinib to gefitinib (LASER301).
Janssen licensed lazertinib from Yuhan in 2018. In 2019, lazertinib combination cohorts were added to the ongoing amivantamab study in EGFRmut NSCLC (CHRYSALIS).
Preliminary data was presented at ESMO 2020 for two cohorts:
• Prior osimertinib, but chemo-naïve (N = 45): 36% ORR
• Tx-naïve (N = 20): 100% ORR
The AE profile warrants some caution, with rash occurring in 85% of pts (including grade 3 in 4%) and infusion-related reactions in 65%.
An ongoing phase 1b/2 study combines lazertinib and amivantamab in EGFRmut NSCLC pts with prior osimertinib and prior platinum-based CT (CHRYSALIS-2). This study could provide a quick-to-market opportunity given the significant unmet need in that setting.
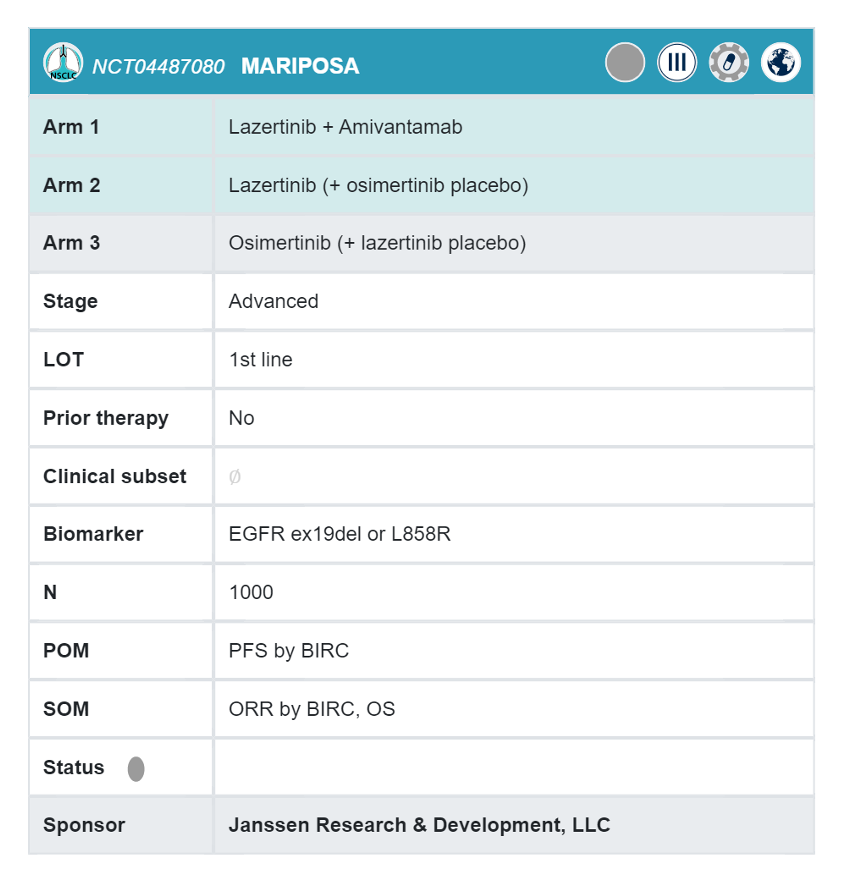
The planned phase 3 MARIPOSA study in tx-naïve, EGFRmut NSCLC compares lazertinib/amivantamab to single agent osimertinib. The study includes a third arm for single agent lazertinib, but this arm is not part of the primary analysis.
Reference
LASER301: (NCT04248829)
CHRYSALIS: (NCT02609776)
CHRYSALIS-2: (NCT04077463)
MARIPOSA: (NCT04487080)
Lee et al., (ASCO 2020: 9572)
Cho et al., (ESMO 2020: 1258O)